- Research Services
- In Vivo
In Vivo Research Solutions
Research Expertise Highlights
In vivo research is at the core of breakthrough therapies and innovative drug development.
Virscio leads the way in delivering precise, reliable, and actionable in vivo research data that drives medical advancements. With over 200 studies completed, we specialize in uncovering insights that reduce risk and optimize therapeutic development. Start your journey towards innovative solutions—contact Virscio today for comprehensive in vivo research services.
Comprehensive In Vivo Capabilities
- Pharmacokinetics & Pharmacodynamics
- Disease Modeling & Efficacy Testing
- Custom Model Development
- Toxicology & Safety Assessments
Advanced Research Techniques
- Behavioral Studies
- DMPK Analysis
- Cutting-Edge Imaging
Why In Vivo Research Matters
In vivo research is essential for translating laboratory findings into therapeutic realities. It provides critical data on drug safety, efficacy, and biological interactions. At Virscio, we understand that successful drug development hinges on the ability to predict how a treatment will perform in real-world scenarios. That's why our in vivo studies are designed to offer a complete view of your drug’s potential, from initial testing to detailed efficacy analysis.

Virscio's In Vivo Research Capabilities
Virscio is adept at conducting a range of in vivo studies across various administration routes. Our team's expertise spans multiple methodologies, ensuring that each study is tailored to the specific needs of the research.
Our In Vivo Research Capabilities include:
- Pharmacokinetics and Pharmacodynamics (PK/PD): We evaluate how drugs are absorbed, distributed, metabolized, and excreted in the body, offering insights that are critical for dosage optimization and therapeutic success.
- Disease Models & Efficacy Testing: Employing a diverse range of disease models, our studies provide comprehensive insights into therapeutic impacts and mechanisms of action.
- Toxicology and Safety Assessment: Our team conducts thorough toxicology studies to identify potential risks, ensuring that your therapeutic candidates meet safety standards before progressing to clinical trials.
- Drug Metabolism and Pharmacokinetics (DMPK): Understand how drugs interact within biological systems to optimize development strategies.
- Custom Model Development: Tailored models that reflect specific disease conditions, enhancing the relevance and accuracy of your research.
Advanced Imaging & Analysis Techniques
Virscio integrates state-of-the-art imaging technologies to monitor real-time biological processes and therapeutic effects. This approach enhances data accuracy, offering deeper insights into drug efficacy and safety:
Optical Imaging: Real-Time Cellular Insights
Get real-time visualization of cellular and molecular interactions in in vivo research. Our advanced optical imaging techniques provide high-resolution data that’s crucial for accurate disease modeling, efficacy testing, and pharmacodynamics assessments. By capturing immediate biological responses, you gain actionable insights to optimize preclinical data and strengthen your regulatory submissions.
MRI & PET: Detailed Anatomical and Functional Assessments
Leverage MRI and PET imaging for comprehensive anatomical and functional evaluations in preclinical studies. These advanced imaging modalities allow you to observe drug distribution, disease progression, and target engagement in greater detail—especially valuable when working with nonhuman primate models. The resulting quantitative data ensures precision and clarity in translational research, paving the way for more informed therapeutic decisions.
Quantitative Analysis: Advanced Data Interpretation
Turn imaging results into robust, actionable insights with our quantitative analysis approach. We integrate pharmacokinetics, pharmacodynamics, and toxicology data to support regulatory submissions and clinical trial readiness. By synthesizing a range of in vivo metrics, we deliver a cohesive, evidence-based narrative around your drug’s efficacy and safety, ultimately accelerating your path to successful therapeutic development.

Why Partner with Virscio for Your In Vivo Research?
Partnering with Virscio for in vivo research means choosing a leader in the field. Our comprehensive approach, combined with a commitment to innovation and accuracy, ensures that your research is not only conducted efficiently but also yields meaningful and reliable results. Our expertise in a variety of models and cutting-edge technologies positions us as the ideal partner for your in vivo research needs.
Unmatched Expertise
Our team brings decades of experience in in vivo modeling, pharmacokinetics, and specialized therapeutic areas. This depth of knowledge ensures precise study design and execution for reliable, translatable results.
Cutting-Edge Technology
From advanced imaging platforms to AI-enhanced data analysis, we stay at the forefront of preclinical innovation. Our commitment to modern tools accelerates research timelines and enhances data quality.
Comprehensive Services
We offer everything from efficacy testing and toxicology assessments to custom disease model development. This one-stop approach streamlines your research process and keeps all critical stages under one roof.
Collaborative Engagement
Our scientists work closely with sponsors, providing clear communication and tailored study designs. We see ourselves as an extension of your team, dedicated to achieving your research goals.
Rigorous Quality Standards
Virscio adheres to strict regulatory guidelines and maintains robust quality systems to ensure each preclinical in vivo study is conducted ethically, accurately, and reproducibly.
Proven Track Record
With 20+ years of experience and over 200 in vivo studies completed, we’ve consistently demonstrated our ability to deliver actionable insights that drive therapeutic development forward.
Contact us today and learn how Virscio can help accelerate your in vivo research.



Ethical Standards & Animal Welfare in In Vivo Research
We uphold the highest ethical standards in every phase of our in vivo research. Beyond adhering to regulatory guidelines and compliance measures (including GLP and AAALAC requirements), our team actively prioritizes animal welfare through humane treatment protocols. We employ the 3Rs (Replacement, Reduction, Refinement) to minimize animal use and optimize study designs, ensuring data integrity while respecting the welfare of all subjects. At every stage—from planning to execution and reporting—we maintain full transparency and integrity, fostering trust with clients and advancing scientific discoveries responsibly.
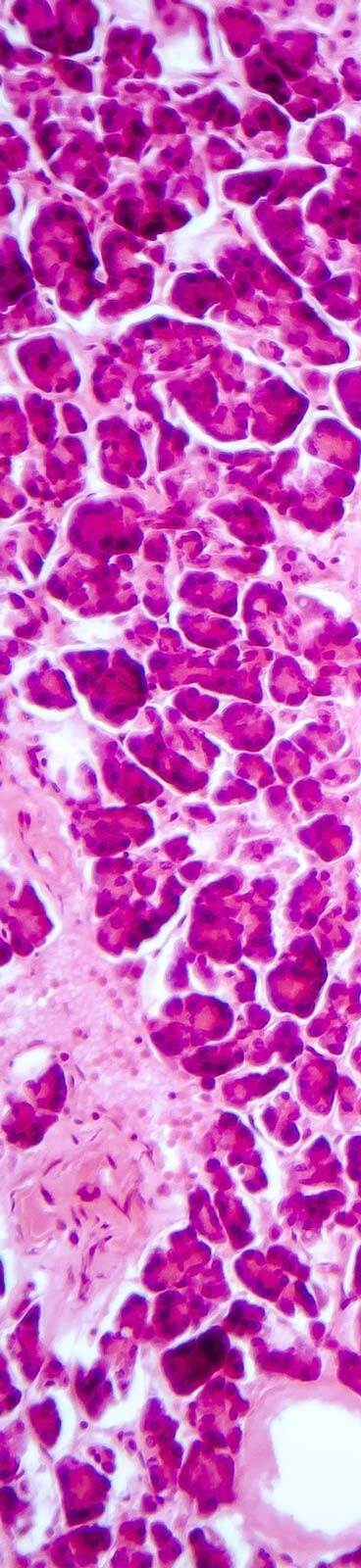
Seamless Transition from In Vivo to Histopathology
Bridging In Vivo Insights and Ex Vivo Precision
At Virscio, our commitment to comprehensive research extends well beyond in vivo testing. We seamlessly integrate ex vivo and histopathological analysis into our workflows, ensuring that each study yields deeper insights into a therapeutic’s mechanism of action, safety, and potential efficacy.
Coordinated Study Design
Our scientists design each in vivo study with downstream analyses in mind, collecting samples and data in a way that optimizes ex vivo evaluation. This reduces the need for repeated experiments and minimizes animal usage, in line with our ethical research principles.
Advanced Histopathological Techniques
We leverage cutting-edge techniques—such as immunohistochemistry, in situ hybridization, and AI-driven image analysis—to provide detailed tissue-level data that complements in vivo observations.
Conclusive Translational Insights
By merging in vivo outcomes with histopathological findings, we offer comprehensive reports that elucidate drug action, potential off-target effects, and efficacy markers. This holistic view accelerates decision-making for sponsors and paves the way for more successful IND submissions.
Why It Matters
In vivo results are most powerful when backed by corresponding tissue analyses, helping you confirm target engagement, understand safety margins, and build a robust translational narrative. Our integrated approach shortens study timelines, drives cost-effectiveness, and delivers a unified dataset, boosting confidence in your development pipeline.
Discover more about our capabilities
Visit our scientific library and get access to a wide range of resources showcasing Virscio’s translational research capabilities, including posters, case studies, journal articles and more!
Frequently Asked Questions
What is in vivo research, and why is it important?
In vivo research involves studying biological processes within a living organism. It provides critical insights into how potential therapeutics behave in real physiological environments, allowing researchers to evaluate drug safety, efficacy, and mechanism of action before progressing to clinical trials.
How does Virscio ensure ethical treatment of research animals?
Virscio upholds strict animal welfare standards, adhering to regulatory guidelines such as GLP and AAALAC requirements. We follow the 3Rs principle (Replacement, Reduction, Refinement) to minimize animal use and refine our study designs, ensuring both scientific rigor and humane treatment.
What types of in vivo studies do you offer?
We conduct a wide range of studies, including pharmacokinetics (PK), pharmacodynamics (PD), toxicology, disease modeling, drug metabolism, and efficacy testing. Our team can also develop custom models tailored to your specific research needs.
Which disease models does Virscio specialize in?
We have extensive experience in a variety of therapeutic areas, including CNS, ophthalmology, cardiovascular, metabolic, and more. Our validated models closely replicate human diseases, ensuring higher translational relevance.
How does Virscio maintain data quality and integrity?
We use rigorous quality control processes and advanced analytical tools throughout our studies. Our protocols ensure consistent data collection, transparent reporting, and compliance with regulatory standards, helping you make informed decisions for your therapeutic pipeline.
Can you accommodate customized study designs or specific client requirements?
Absolutely. We collaborate closely with clients to design and execute studies that meet their unique objectives, whether it’s optimizing dosing, investigating specific pharmacological pathways, or addressing complex regulatory requirements.
How long does an in vivo study usually take at Virscio?
Timelines vary depending on the scope and complexity of the study. We work with clients to set realistic goals and milestones, providing regular updates and delivering results efficiently without compromising on scientific rigor.
What technologies do you use to enhance in vivo studies?
Our research solutions integrate state-of-the-art imaging (MRI, PET, optical imaging), AI-enabled data analysis, and advanced molecular biology techniques. These tools help us collect and interpret data with greater speed and accuracy.
Do you handle the regulatory aspects of preclinical research?
Yes. Our team is experienced in navigating regulatory guidelines and preparing the necessary documentation for IND-enabling studies. We ensure that all preclinical data meets the standards required by regulatory agencies worldwide.
How can I learn more or get started with Virscio’s in vivo research services?
You can reach out via our Contact Us page or schedule a consultation. We’ll discuss your research goals, propose a tailored study design, and guide you through the entire preclinical research process.